
Ionization Energy Periodic Table Trend. As an element approaches the top
Electron affinities follow the same trends as the ionization energy across

The ionization energy of an atom is the energy required to remove an
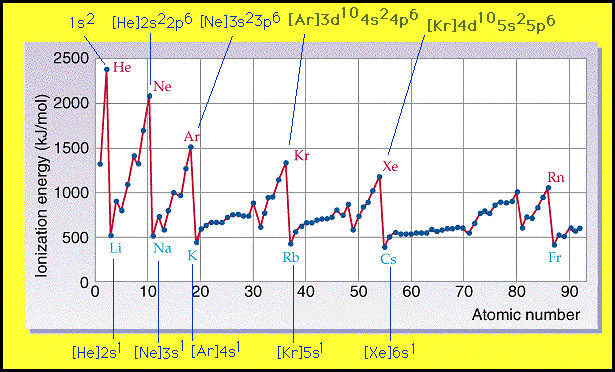
The ionization energy of an atom is the energy required to remove an
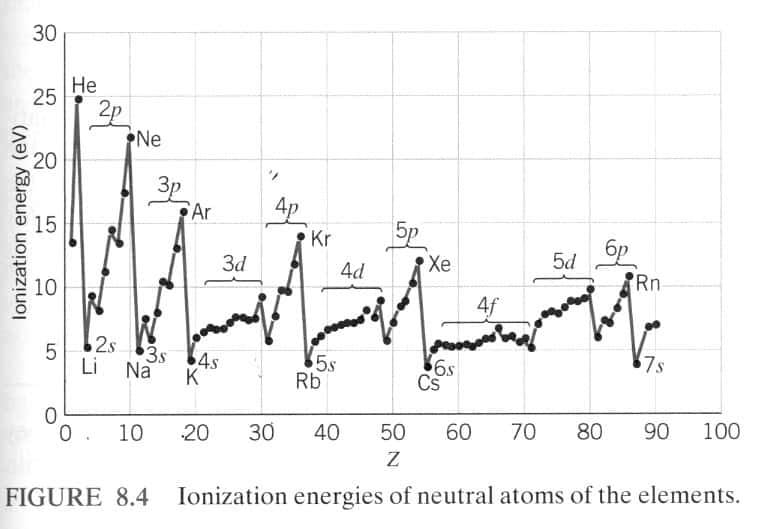
This is because the electrons enter a new energy level as one moves down a

Discuss Periodic Trends: Atomic radius · Ionization Energy

Ionization Energy Trend IK.png

periodic table with the periodic trend of ionization energy (incresing up

Electronegativity, Ionization Energy & Electron Affinity: Opposite Trend of

SECOND IONIZATION ENERGY. X1+(g) + ENERGY ---> X2+(g) + e1-

Ionization Energy Trend. Formation of atomic size Using the ionization

Ionization Energy Trend Periodic Table. Expect the periodic table ionization

Ionization Energy Down a Family (Group) (Top to Bottom) Decreases.

Ionization energies are very much a function of the electronic structure of

Ionization Energy Trend. Periodic-table-ionization-energy- details of

ionization energies periiodic trends. Some points to note:

Note a very fast decrease in the opacity with increase in the ionization

Periodic Trends - Atom size, Ionization Energy, Summary
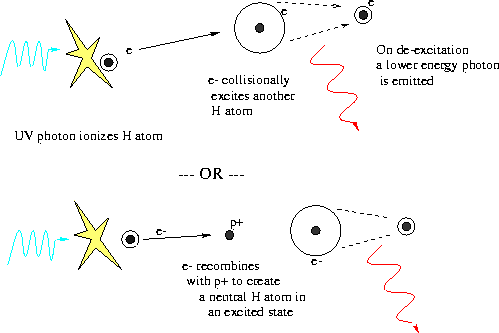
IONIZATION ENERGY TREND

ionization energy: increases from left right arrow
